Impact
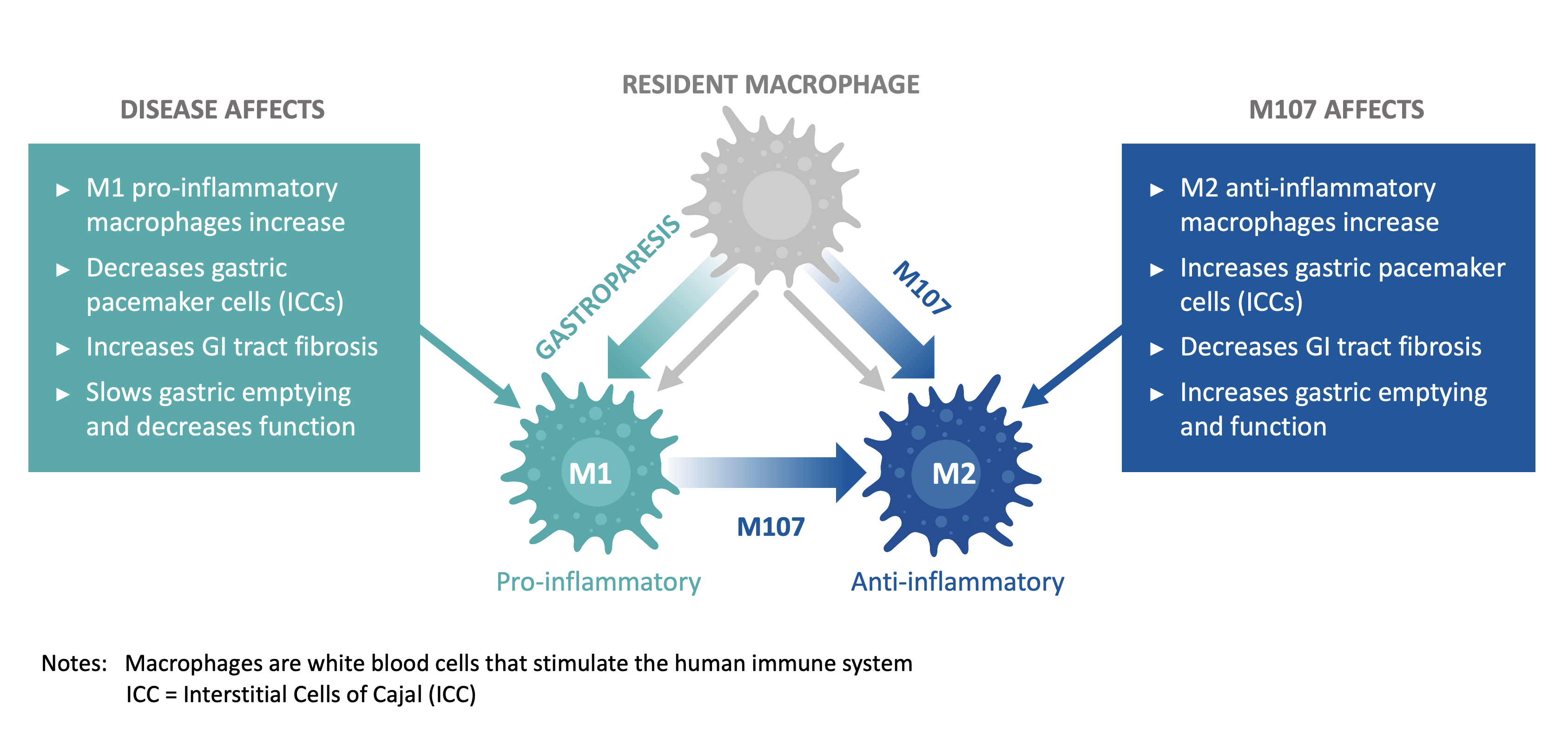
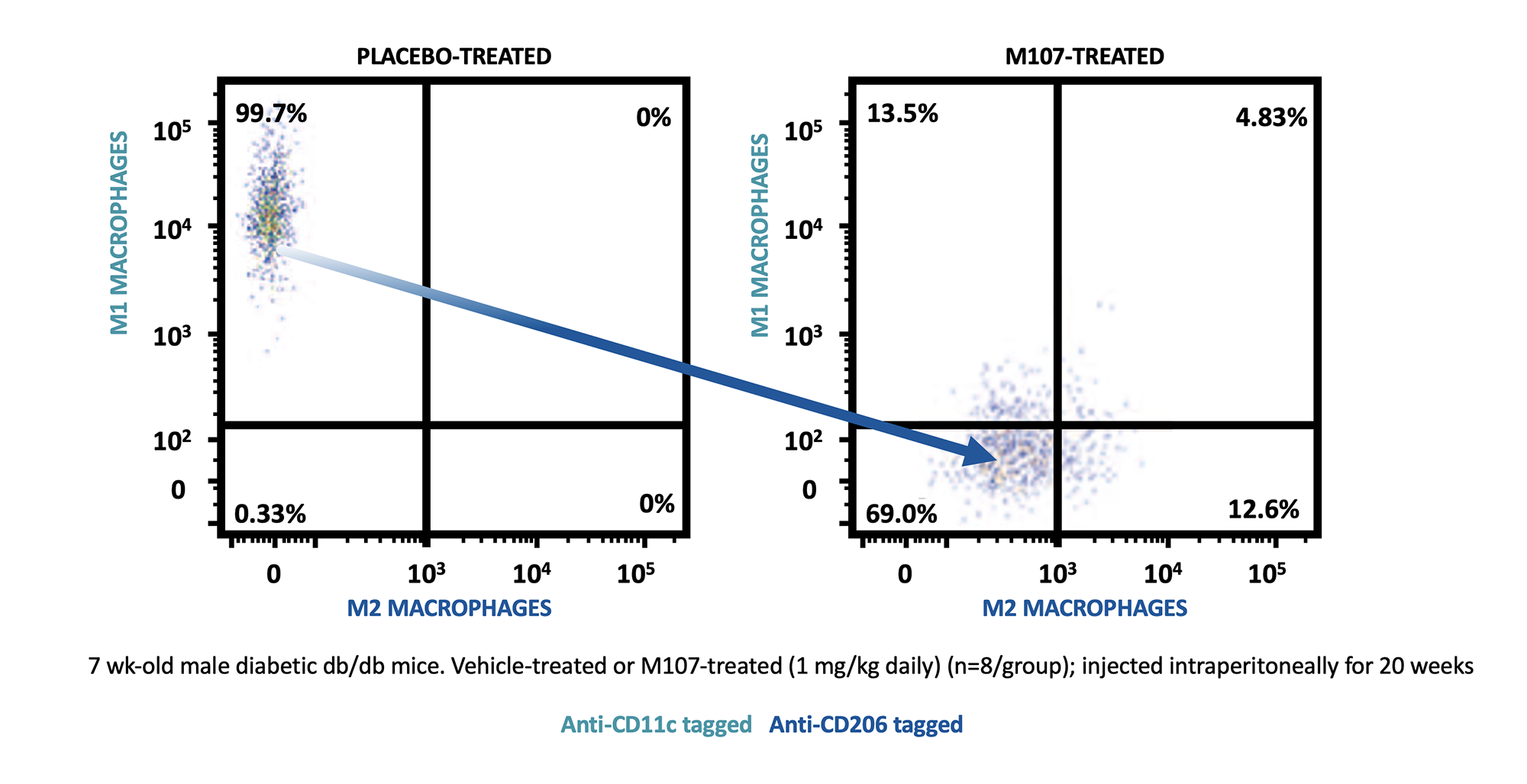
In vivo testing of M107 has demonstrated excellent on-target effects promoting increased expression of M2 macrophages, decreased number of M1 macrophages, and reduction of inflammatory markers. Human testing has shown a much improved off-target safety profile for M107 compared to earlier PPARγ molecules.