Aclipse focuses on neuromuscular diseases that are strongly impacted by molecular pathways that regulate cellular stress, protein misfolding and inflammation. M107 may be the first potential disease-modifying drug candidate for the treatment of gastroparesis. M102 may be the first precision medicine for the treatment of amyotrophic lateral sclerosis (ALS), also known as motor neurone disease (MND) or Lou Gehrig’s disease.
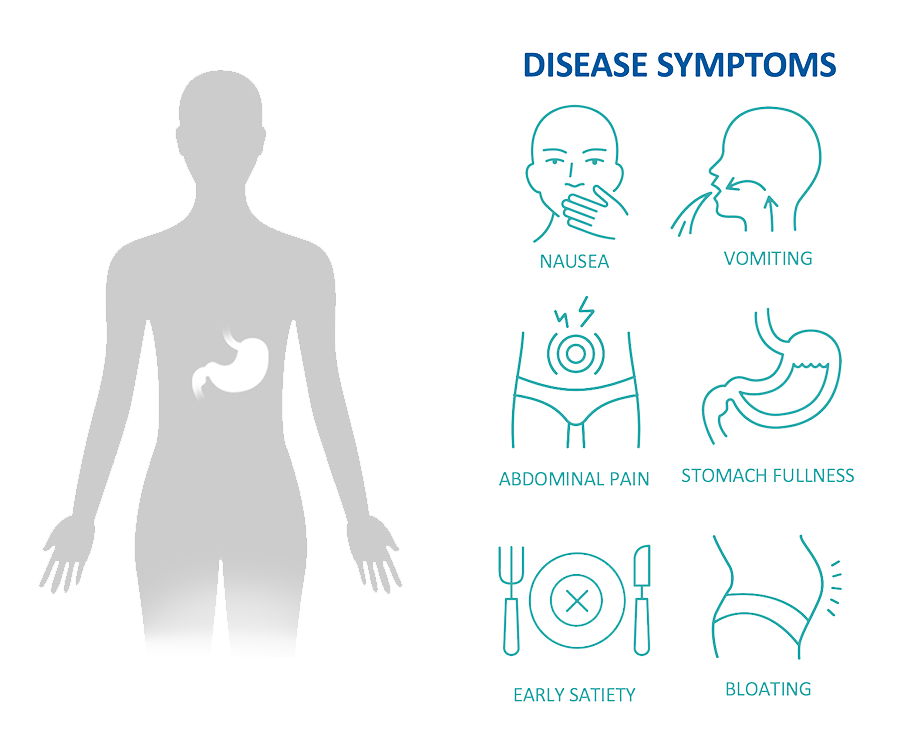
Gastroparesis (GP) is a serious disease affecting stomach nerves and muscles resulting in stomach paralysis and delayed stomach emptying of foods. Disease symptoms include nausea, vomiting, abdominal pain, stomach fullness, early satiety, and bloating. It is a chronic, often poorly managed condition with all approved drugs treating only symptoms of the disease. GP afflicts approximately 600,000 US patients with a high hospitalization rate. Over 75% of patients classify the disease as severe or very severe and 65% of patients are dissatisfied with current symptomatic treatments.
In addition, growth of GLP-1 agonists (Ozempic®, Mounjaro™, Wegovy® and Zepbound™) for the treatment of weight loss creates a significant upside opportunity for M107. GLP-1 drugs are projected to treat as many as 333 million U.S. patients by 2030. Gastrointestinal side effects are the most common adverse events. Gastroparesis is reported in 0.9% to 5.6% of GLP-1 patients depending upon the specific GLP-1 drug.
Amyotrophic lateral sclerosis (ALS) is a fatal, incurable and debilitating neurodegenerative condition that results in progressive degeneration and death of nerve cells and the eventual loss of the ability to eat, speak, move and breathe. Life expectancy is 2 to 5 years post-diagnosis. The heterogeneity and complexity of these disorders have proved to be major challenges for drug development. There is an urgent need for effective neuroprotective therapies.